SCUBA DIVING EXPLAINED Questions and Answers on
Lawrence Martin, M.D. Copyright 1997
|
||||||||||||||||||||
|
Home Brief History of Diving Glossary
|
SECTION FEffects of Unequal Air Pressures While Scuba Diving: Ear Squeeze, Sinus Squeeze, Air Embolism and Other Forms of BarotraumaWHAT DOES BOYLE'S LAW PREDICT ABOUT UNEQUAL PRESSURES? Once again we come to all-important Boyle's law: for a given mass of gas at a constant temperature, the product of pressure (P) and volume (V) is constant (K): P x V = K. We have already seen how, for the breath-hold diver, Boyle's law predicts that compressible air spaces will shrink on descent and re-expand on ascent, and that the situation is different for scuba divers because compressed air is continuously inhaled. Even if the scuba and breath-hold diver go to the same depth and spend the same amount of time under water (e.g., one minute), the effects of water pressure are radically different on the two divers. Because tank air is inhaled at the ambient pressure, the scuba diver's lungs and other compressible air spaces do not shrink. Consider that all the body's air-containing spaces are in contact with inhaled air. At the same time, there is a natural tendency for air anywhere in the body - in the lungs, middle ears, sinuses and other spaces - to diffuse into the blood. As air is absorbed into the blood it is replenished by fresh air inhaled from the scuba tank. If the absorbed air was not replenished the air spaces would, over time, shrink and close. (This in fact happens in some lung diseases. Any part of a lung that becomes plugged, such as from mucus or a tumor, will shrink completely until it becomes airless.) Shrinkage and closure of an air space will not happen as long as it is in contact with a fresh supply of air. Does Boyle's law still apply to such spaces? Unequivocally, yes. When fresh air enters the diver's lungs it is at the same pressure as the surrounding water pressure. This pressure, of course, is higher than at sea level and so the air is correspondingly denser. As long as the diver continuously breathes from the scuba tank, the density of inhaled air will change with ascent and descent. For this reason the "given mass of air" stipulated in Boyle's law, which is really the number of air molecules, changes as the depth changes. On descent the "given mass" increases as the inhaled air becomes denser; on ascent the "given mass" decreases as the inhaled air becomes less dense. Stated another way, when breathing compressed air under water, the actual number of air molecules, in the lungs and all other air spaces, increases on descent and decreases on ascent. Assume two people dive from a boat to a depth of 99 feet; one diver holds his breath and the other diver uses compressed air (scuba). Also assume that the lungs of each diver contain 10 billion air molecules and the lungs behave like balloons. Table 1 shows the physical changes in air in the lungs of each diver at 33, 66 and 99 fsw. (Actually, the breath-hold diver's lungs, being tethered by a rib cage, don't behave exactly like balloons. Also, some gas exchange takes place even with breath holding, since oxygen is taken up from air in the lungs while a smaller amount of CO2 is added to that air; as a result, the number of molecules does not remain exactly the same.)
For the breath-hold diver, the mass of gas (the number of molecules) in the air spaces remains about the same during the dive, so as water pressure increases the lungs must shrink (Boyle's law). For the scuba diver, however, the mass of gas (number of molecules) increases along with the increase in water pressure, so the lungs do not shrink. Thus the lungs (and other air spaces) of a scuba diver at 33 fsw contain twice as many air molecules as at sea level, and also twice as many air molecules as a breath-hold diver at the same depth (allowing for slight variation due to absorption of oxygen into the blood). Since the volume of air in the scuba diver's lungs doesn't change, the density of air (how close the molecules are to one another) must be greater (see Table 1). At 99 fsw the breath-hold diver's lungs contain about the same number of molecules as on the surface, but in only 1/4 the volume; hence the air, being compressed by the increased pressure, is four times denser than on the surface. At 99 fsw the scuba diver's lungs are filled with air just as dense, but since there are four times the number of molecules as on the surface the lung volume is preserved (Table 1). As long as there is good communication with inhaled air, the scuba diver's compressible air spaces will fill up with the air extra molecules and will not shrink.
TEST YOUR UNDERSTANDING Answers Everything described so far fits with Boyle's law. 'Pressure times volume' is a constant value when the mass of gas (number of gas molecules) is fixed; it is not constant if the gas density changes, as occurs with changes in depth, because then the mass of gas in any space also changes. The most critical result of Boyle's law occurs when the mass of gas is fixed, as would occur in the lungs of a scuba diver if breath is held. If the scuba diver was to breath-hold and ascend, the fixed mass of gas at the time of breath-hold would inexorably expand in volume (as predicted by Boyle's law) until the diver exhaled or the lungs ruptured. This, of course, is why a breath-hold ascent from any scuba dive is so dangerous.
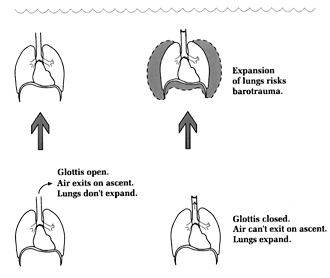
TEST YOUR UNDERSTANDING Answers WHAT IS THE DIFFERENCE BETWEEN SCUBA DIVING WITH THE GLOTTIS CLOSED AND OPEN? The glottis is the voice box, located just under your chin inside the neck. Also called the larynx, it leads directly into the trachea (the "windpipe") which then leads to the lungs (see Chapter 3). We "close" the glottis when we hold our breath, which is why you can't speak and hold your breath at the same time. (The universal sign of someone choking on food is fingers to the throat and inability to speak.) What happens when the glottis is open or closed relates directly to the difference between compressible and non-compressible structures, as was discussed in Chapter 5. Non-compressible structures include the blood, bones and all solid organs; compressible structures include the lungs, sinuses, middle ear, and the hollow organs such as the stomach and intestines. Figure 1 shows effects on the lungs from increased ambient pressure when the glottis is closed ("Closed"), and when air is replenished from scuba apparatus ("Scuba"). A scuba dive with breath held (glottis closed) is tantamount to a breath-hold dive (Table 1); the lungs will be squeezed by the increased ambient pressure. An initial volume of 6 liters (e.g., after taking in a regular breath) could theoretically shrink to only one 1.2 liters at a depth of 132 feet (Figure 1). However, the scuba diver has the option (which should always be exercised) to continuously breathe compressed air, in which case lung volume will stay the same at all depths (allowing for slight variation with regular breathing). In both situations (glottis closed and breathing compressed air) the density of air in the lungs will increase with increasing depth. Air in the tank is highly pressurized, approximately 3000 psi (equivalent to 204 atmospheres) for an 80 cu. ft. tank filled to capacity. The two-stage regulator allows compressed air to be inhaled at the ambient pressure, so the scuba diver can maintain normal lung volume at all depths. (The increased density of the inspired air at depth is seldom great enough to impair work of breathing during recreational diving.) Tank pressure should be at least 500 psi before beginning any ascent (ideally, one should arrive at the safety stop with no less than 500 psi). Five hundred psi are equivalent to 34 atmospheres. Since the maximum RSD depth of 130 feet equals only about five atmospheres, there should always be a large gradient for air to flow from the tank to the diver's lungs.
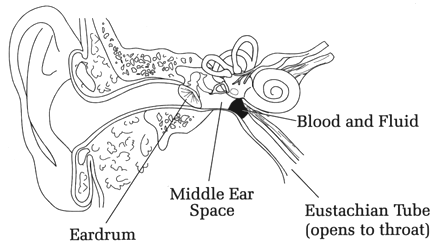
Figure 1. Effects on lungs with glottis open and closed. WHAT IS BAROTRAUMA? 'Baro' refers to pressure (e.g., a barometer is an instrument for measuring pressure). Barotrauma is physical damage to any part of the body as a result of unequal air pressures; it is from either compression or expansion of a body part. The tissues may rupture, blood supply may be compromised, or swelling may occur. All conditions discussed in this chapter are forms of barotrauma. In the case of diving, the unequal pressures are between some cavity of the body (the middle ear, the sinuses, the lungs, etc.) and the ambient air pressure. The consequences of barotrauma may range from mild discomfort in the affected area (e.g., ears or sinuses), to various levels of pain, to rupture of an organ, such as the ear's tympanic membrane or a part of the lung. Rupture of an organ while diving is particularly hazardous because of the risk of drowning. Barotrauma is one of three pressure-related problems encountered in scuba diving; the other two are decompression sickness and nitrogen narcosis (Table 2). Barotrauma problems (including the most severe, arterial gas embolism), are physiologically explainable by Boyle's. Non-barotrauma, pressure-related problems are physiologically explainable by the laws of Dalton and Henry, as will be discussed later. WHAT BAROTRAUMA PROBLEMS CAN OCCUR ON DESCENT? Most problems on descent relate to inadequate communication between the upper airway and the middle ears and sinuses. The most frequently en-countered problem on descent is middle ear "squeeze," an ear discomfort or pain from shrinkage of the middle ear space (Figure 2). This problem may begin at only a few feet depth. The earliest symptoms are similar to the "ear stuffiness" we sometimes feel when a plane rapidly descends. Unchecked during a dive, the feeling can rapidly progress to actual pain and ear damage. The pathologic result of continued squeeze in any space is engorgement of the mucosal lining, swelling, fluid buildup and finally hemorrhage into the space. In unchecked middle ear squeeze, the tympanic membrane or round window can collapse inward and burst, leading to extreme dizziness (vertigo) and an emergency situation. In a compilation of 1001 disorders referred to an otolaryngology (ear, nose, throat) diving specialist, the middle ear accounted for 399 (40%); 314 of the total (31%) were manifested by pain on descent (Roydhouse 1985). WHAT IS THE CAUSE OF MIDDLE-EAR SQUEEZE AND HOW CAN IT BE PREVENTED? Squeeze on the middle ear is prevented by making sure inhaled compressed air travels from the back of the nose (nasopharynx) into the middle ear spaces. The only route of passage into the middle ear is through a tiny, compressible canal called the eustachian tube (after its discoverer, the Italian Bartolommeo Eustachi, 1524-74). Anatomically, this is a soft and flexible canal that functions as a one-way flutter valve; it easily opens up when pressure in the middle ear is higher than in the nasopharynx, but tends to close shut when pressure in the nasopharynx is higher than in the middle ear. As a result, gas flow is passive from the middle ear to the nasopharynx on ascent (you don't have to think about it), but "active" on descent (you have to make it happen).
Figure 2. Middle ear squeeze. The external ear canal leads to the flexible tympanic membrane or eardrum, which is exposed to the ambient pressure. Behind the ear drum is the middle ear air space, which will be compressed at depth unless pressure is equalized, via the eustachian tube, with inhaled air. If the eustachian tube is blocked Ä as may happen without equalization after descending just a few feet Ä fresh air cannot enter the middle ear space and the ear drum will bulge inward, causing pain. If the diver descends too quickly without equalization, the tympanic membrane can rupture. If the diver continues to descend slowly without equalization, blood and fluid from surrounding tissue will be forced into the middle ear space. Thus the diver has to consciously work to keep the eustachian tube open on descent, or else it will seal shunt and prevent compressed air from reaching the middle ear. This is done by one of several maneuvers, including blowing against a closed mouth and nose, swallowing, yawning, or the Valsalva or Frenzel maneuvers. (The Valsalva is a forced exhalation with nose pinched, lips closed against mouthpiece, glottis open. The Frenzel is accomplished with nose pinched and lips closed against the mouthpiece; the back of tongue is thrust against soft palate, gently pushing air through the eustachian tubes.) Whichever method is used, it must be done frequently on descent because at some point no maneuver will work; this is the situation when the pressure keeping the tube shut is too great. If the pressure gradient across the tube (nasopharynx to middle ear) exceeds 90 mm Hg - a gradient reached at only about 4 feet depth - none of the maneuvers will open the eustachian tube and the diver must ascend to relieve the pressure. Scuba divers are universally taught to prevent middle ear squeeze by forcing air through the eustachian tubes before symptoms occur, just before or at the beginning of descent and then every few feet. "Equalize early and often" is the universal advice. WHAT IS THE TREATMENT OF MIDDLE EAR SQUEEZE? Treatment of middle ear squeeze depends on its severity. Mild cases often to respond to decongestants. Antibiotics may be indicated if there is tympanic membrane rupture, but such a problem should be referred to an otolaryngologist. In all cases diving should be avoided until the ear has returned to normal. WHAT OTHER FORMS OF BAROTRAUMA OCCUR ON DESCENT? The inner ear can also be affected, with rupture of the round or oval windows, cochlear damage and permanent hearing loss. Tinnitus, vertigo, and deafness after a dive are the classic symptoms of inner ear barotrauma. In such cases antibiotics and bed rest are indicated, with surgical repair if there is no improvement (Neblett 1985; Davis & Kizer 1989). External ear squeeze may occur if the ear canal is blocked with ear wax or ear plugs; divers should never wear ear plugs for this reason. Another common cause of external ear squeeze is a tight-fitting wetsuit hood. The sinuses have no natural one-way valve by which to vent expanding air, but as long as there is no sinus blockage the diver can avoid "sinus squeeze." Sinus squeeze is accompanied by a painful feeling behind the cheekbones, between the eyes, in the upper teeth area, or over the forehead. A blockage from outside the sinuses (as from nasal polyps) may produce sinus squeeze on descent, as air cannot enter the sinuses and equalize the pressures. In severe cases the diver can experience sinus hemorrhage and a bloody nose. Decongestants and analgesics are used for mild cases of sinus squeeze. Antibiotics may be needed if there is evidence of fluid or blood in the sinus cavities. Diagnosis of this problem may require a CT scan of the sinuses. WHAT BAROTRAUMA PROBLEMS CAN OCCUR ON ASCENT? Closed air spaces pose a serious threat on ascent from a dive. Boyle's law predicts that closed air spaces will expand as the ambient pressure decreases on ascent. As long as the eustachian tube and sinus passages are not blocked, the middle ears and sinuses will vent expanding air into the nose, from where it will be exhaled along with expanding air from the lungs. "Reverse squeeze" can occur if the eustachian tube and nasal passages are blocked on ascent. This is much less common than squeeze on descent, for if compressed air can get into these spaces it usually can get out. However, sometimes with an infection divers can get air into the spaces but then the air is blocked coming out. The sinus and/or middle ear spaces attempt to expand as the air in their spaces expands; the result is pain on ascent. In a worst-case scenario, middle ear expansion without adequate venting could lead to rupture of the tympanic membrane outward, resulting in severe pain, vertigo, and drowning. For these reasons people with upper respiratory infection, sinus or nasal congestion, or middle ear infection should not dive until the problem is resolved. Alternobaric or "pressure" vertigo is a feeling of disorientation and spinning caused by a sudden, and unilateral, pressure difference between the middle and inner ear (Farmer 1990). It usually occurs during or immediately after an attempt to equalize middle ear pressure by the Valsalva maneuver. Alternobaric vertigo has been described on both ascent and descent, but is more common on ascent. If symptoms persist on the surface treatment is with decongestants, although surgery (a myringotomy Ä placing an opening in the tympanic membrane) may be necessary. Any unvented space can cause discomfort or pain on ascent. Gastric discomfort is rare because of the ease of venting the stomach and intestines but does occur (Weeth 1965; Edmonds 1976). Barodontalgia (tooth pain), usually from an improperly or incompletely filled tooth, can occur on ascent when air in the cavity expands.
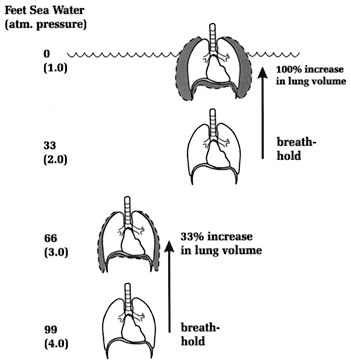
TEST YOUR UNDERSTANDING Answers WHAT IS PULMONARY BAROTRAUMA? Pulmonary barotrauma is any damage to the lungs from unequal air pressures. The greatest danger to the diver on ascent is from pulmonary barotrauma, a risk greatly increased if breath is held. The consequence can be serious, even fatal. The potential for pulmonary barotrauma is why the first rule of scuba diving is never hold your breath. Continuous breathing allows the lungs and communicating spaces to maintain equilibrium with the increased ambient pressure. If breath is held on ascent, air in the lungs will try to expand against an essentially fixed chest volume; depending on the vertical distance of a breath-hold ascent, the result can be anywhere from simple over inflation to lung rupture and passage of air into the blood stream. A maximum safe rate of ascent in RSD is considered to be 60 feet a minute (the slower the better); at all times the diver should continuously breathe. Should a diver run out of air and have to make an emergency ascent, proper technique requires continuous exhalation under water (by saying "Ahh...") in order to vent the expanding lung volume. This maneuver keeps the glottis open and allows continuous exhalation of expanding air, so the lung volume does not increase. For an equivalent change in depth the risk of expansion barotrauma is greatest near the surface, a fact explainable by Boyle's law. A breath-holding scuba diver rising from 33-feet depth to the surface experiences a change in ambient pressure from two to one atmospheres absolute; if the lungs fully expand within the chest cavity lung volume will try to double. By contrast, a 33-foot rise from 99 to 66 feet depth (i.e., from 4 to 3 atmospheres) would maximally increase a breath-holder's lung volume only 33 percent, posing much less risk of barotrauma (Figure 3). The Greatest Risk of Expansion Barotrauma is Near the Surface.Barotrauma correlates with both increase in pressure in the lungs and 'over stretching' of the lung tissue. Experiments in dogs undergoing rapid ascent in a chamber showed that the lungs can withstand much higher pressures (before barotrauma occurs) if the chest cavity is bound and 'over stretching' is prevented (Schaefer 1958). Although both over stretching of lung tissue and the pressure of expanding air are factors favoring lung trauma, pressure seems to be the major one. The pressure difference across the lungs (from inside to outside) that is the threshold for experimental barotrauma is about 80 mm Hg; this can occur with a breath-hold ascent from only four feet! The pressure difference (and risk of barotrauma) is obviously much greater with breath-hold from greater depths. During a breath-hold ascent from 33 feet the lung volume would try to double, almost guaranteeing barotrauma if breath were held at or near the diver's total lung capacity (Figure 3). If the lungs could not vent expanding air they would be subjected to a distending pressure of nine times the barotrauma threshold!
Figure 3. Ambient pressure and percentage change in lung volume with equivalent depth change (33 feet). In each instance the diver is breathing compressed air at the point of breath-hold. Pulmonary barotrauma usually manifests immediately after ascent but may be delayed for several hours. It may also recur after an initial period of improvement (Krzyzak 1987). People suffering pulmonary barotrauma should be treated with a high inspired oxygen concentration, 100% if available. Oxygen "denitrogenates" the blood and hastens absorption of bubbles (see Section H).
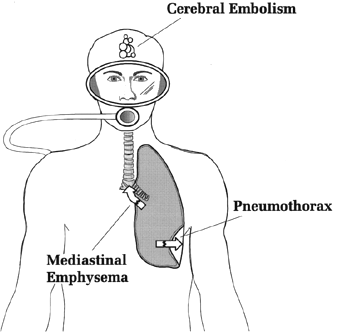
TEST YOUR UNDERSTANDING Answers HOW DOES PULMONARY BAROTRAUMA OCCUR? The presumed mechanism of pulmonary barotrauma is as follows. With ascent, if the expanding air cannot be properly vented, the lungs (or some portion of them) expand in response to the increase in pressure; if they expand too much, individual alveoli are prone to rupture. If the lungs are structurally normal, i.e., there are no blebs, bullae, or areas of abnormal tissue (which are more prone to rupture), barotrauma should not occur until a transpulmonary pressure of around 80 mm Hg is reached (Schaefer 1958). Above 80 mm Hg the alveoli are prone to tear and vent air into the surrounding space (called the interstitial space). This transpulmonary pressure should not occur in healthy lungs unless breath is held on ascent. From the interstitial space, escaped air can take one of three paths (Figure 4): between the two lungs (mediastinal air), around one of the lungs (pneumothorax), or into the blood stream (air embolism) (Macklin 1944). 1) Escaped air can dissect along tissue layers into the area known as the mediastinum, the large space between the two lungs. Once in the mediastinum, the air can go into spaces around the heart (but not in it), into the neck, and into spaces around the abdominal organs. 2) Escaped air can rupture through the visceral pleura (thin membrane that lines the lungs), resulting in a pneumothorax, which is an abnormal air collection between the chest wall and the lung. This air collection can compress or collapse the lung. 3) Escaped air can enter the pulmonary veins, from where it can travel to the arterial circulation as an air embolism (traveling air bubbles). This is by far the most serious complication of a ruptured lung, since the air embolism can block blood vessels to the brain or heart and be fatal.
Figure 4. Three pathways air can take once there is a rupture of lung tissue. WHY DOES PULMONARY BAROTRAUMA OCCUR? There is no doubt that pulmonary barotrauma results from unequal air pressures across the lung. But why does it occur in some people and not others. Is it always from a breath-hold ascent? Although breath-hold ascents account for some cases, there are also cases of barotrauma where the divers are certain they never held their breath. There are two explanations for this latter group. First, some divers probably have abnormal lungs and don't know it. Such changes as subpleural blebs and bullae (abnormal air pockets in the lungs) can often be demonstrated by chest CT scanning or even a plain chest x-ray in people with no respiratory symptoms or problems. After one diver suffered major barotrauma, a chest x-ray that was done before the dive was reviewed; it showed a large bulla, or abnormal air space with thin walls. Probably a certain percentage of people have such "weak lungs" (for want of a better term); these weak lungs may cause them no difficulty except when exposed to slight pressure changes that would not affect normal lungs. Still, there are apparently other divers with completely normal lungs, who are confident breath was not held, yet who still suffered pulmonary barotrauma. These events are difficult to explain, and are fortunately rare (as is pulmonary barotrauma in general). Pulmonary barotrauma remains a definite, albeit small, risk of scuba diving. A hyperbaric chamber is not used for pulmonary barotrauma unless there is suspicion of air embolism (discussed below). Chest tube placement for pneumothorax follows the same guidelines as without diving. Pneumothorax is particularly dangerous if the patient is to be transported by air or receive hyperbaric therapy (which might be needed for decompression sickness or air embolism, not the pneumothorax). The decreased barometric pressure of altitude, as well as the decompression phase of hyperbaric therapy, will further expand the pneumothorax space and increase the risk of compressing the lung (and, if very severe, the heart). WHAT IS ARTERIAL GAS EMBOLISM? Arterial Gas Embolism (AGE) is the most serious medical problem related to pulmonary expansion barotrauma. It is a major cause of sport diving deaths (Arthur 1987; Kizer 1987). AGE accounts for about 40% of the case load at active dive accident treatment centers in the U.S. (Kizer 1987). AGE is thought to occur when alveoli rupture and air enters the pulmonary veins, from where it travels through the left heart chambers and into the arterial circulation. Despite this presumed mechanism AGE victims usually do not usually show obvious evidence of lung rupture when their chest x-rays are examined (Kizer 1987; Gorman 1989; Williams 1990). AGE characteristically manifests within minutes of surfacing. In cases of AGE reported to the national Divers Alert Network in 1988, 78% had their first symptoms within five minutes and 88% within 10 minutes (DAN 1988). The early onset of symptoms is in contrast to decompression sickness (discussed in Section G). Decompression sickness is caused by a different type of bubbles (pure nitrogen) and causes symptoms that come on more gradually than AGE. The brain is the most commonly affected major organ in AGE, although air emboli can also block coronary arteries and lead to heart attack (Kizer 1987). Cerebral air embolism can present with sudden unconsciousness or acute neurologic deficit (stroke). Other neurologic symptoms include severe headache, difficulty speaking, and visual loss (Dick 1985). The major alternative diagnosis to AGE is decompression sickness but treatment is the same for both conditions: recompression in a hyperbaric chamber. WHAT IS TREATMENT OF AGE? First aid treatment of suspected AGE requires putting the patient in comfortable position; usually this will be supine and flat. The head-lower-than-the-body position (so-called Trendelenburg position) was once thought to prevent embolism to the brain, but this has not been substantiated and is no longer recommended (Butler 1988). Other first aid includes administering a high concentration of oxygen (see Section H) and plenty of fluids. Arrangements should be made to transport the victim to the appropriate medical facility (See Appendix A). Although symptoms of AGE may improve after first aid, recompression in a chamber is still considered mandatory (Kindwall 1983; Kizer 1987; Green 1987), for two reasons. First, patients with AGE can relapse after initial improvement. Second, AGE may be accompanied by decompression sickness, a condition more insidious in onset and which also responds to recompression therapy. One review found a 12% prevalence of both conditions in seriously ill patients referred for recompression therapy (Green 1987). Once AGE is suspected, the patient should be referred to a hyperbaric chamber.
Answers to TEST YOUR UNDERSTANDING 1. 50 billion molecules; volume of 8 liters. 2. Number of molecules would stay about the same; density would decrease with ascent; volume of air would increase with ascent. 3. d. Her lung would attempt to expand to three times their initial volume, to 18 liters; this is an impossibly large lung volume so there would be lung rupture become she reached the surface. 4. a, b, c 5. a or b 6. c. Rapid up and down motion increases risk of pulmonary barotrauma if breath is held, which it might be in a stressful situation. If breath is not held, mild, non-sustained coughing should not lead to barotrauma. 7. c. 8. a. Boyle's law. The other laws have nothing to do with the pulmonary barotrauma that leads to arterial gas embolism. 9. a and b. Note that the lungs do not have to be weak or diseased for arterial gas embolism to occur. If breath is held on ascent, normal lungs can rupture and lead to air embolism. At the same time breath does not have to be held for embolism to occur; under some conditions weak or diseased lungs (as from asthma or blebs) can spontaneously rupture. 10. False. Arterial gas embolism is due to bubbles of air; decompression sickness is due to bubbles made up, at least initially, of almost pure nitrogen. 11. False. They may be treated the same (with recompression in a hyperbaric chamber), but the causes are different. 12. c and e. The other conditions are not accompanied by bubbles in the blood and tissues, and would not respond to recompression therapy. REFERENCES AND BIBLIOGRAPHY Quoted sources and general references are listed by section or sections, in alphabetical order. An asterisk indicates references that are especially recommended. Medical textbooks and journal articles can be obtained from most public libraries via inter-library loan. For a list of companies that distribute free catalogs of diving books and videos, see Appendix D. SECTION F. Effects of Unequal Air Pressure Underwater: Ear Squeeze, Sinus Squeeze, Air Embolism and Other Forms of Barotrauma See references for Section G. |