SCUBA DIVING EXPLAINED Questions and Answers on
Lawrence Martin, M.D. Copyright 1997
|
||||||||||||||||||||||||||||||||||||
|
Home Brief History of Diving Glossary
|
SECTION GEffects of Increased Dissolved Nitrogen From Scuba Diving: Decompression SicknessWHAT HAPPENS TO INHALED AIR AT DEPTH? Dalton's Law states that the total pressure of a gas is equal to the sum of pressures of its individual components. In the case of air:
where P(t) is the total air pressure, PO2 and PN2 are partial pressure of oxygen and nitrogen, respectively, and P(x) is the partial pressure of remaining gases (less than 1% of air). For example, at sea level the total air pressure is 1 atm. or 760 mm Hg. Of this total air pressure, 21% (or .21) is from oxygen, 78% (.78) from nitrogen, and 1% (.01) from other gases. The percentage of an individual gas times the total air pressure gives the pressure of that component gas. Thus, at sea level:
Dalton's law states that the individual gases of any gas mixture will have the same pressure alone or as part of the mixture. Thus, at sea level, the oxygen component of air by itself will support a mercury column 159.6 mm high, and the nitrogen component will support a mercury column 592.8 mm high. At depth all pressures increase, for both air as a mixture and for its component gases. For example, a doubling of ambient air pressure, which occurs at just 33 fsw, will double the partial pressure of oxygen, nitrogen, and other component gases. At 66 fsw, the ambient pressure is tripled, along with the partial pressure of oxygen, nitrogen and other gases inhaled at that depth.
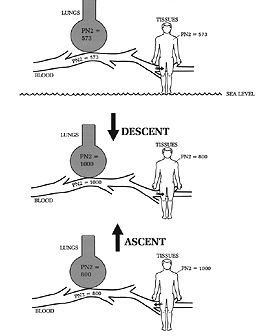
TEST YOUR UNDERSTANDING Answers HOW DOES THE INCREASED PRESSURE AT DEPTH AFFECT GAS IN THE BODY? The increased pressure of each gas component at depth means that more of each gas will dissolve into the blood and body tissues, a physical effect predicted by Henry's Law. To review, Henry's law states that the amount of gas dissolving into any liquid or tissue with which it is in contact is proportional to the partial pressure of that gas (Chapter 4). Inhaled gases are in close contact with blood entering the lungs. Hence, the greater the partial pressure of any inhaled gas, the more that gas will diffuse into the blood. Together, Boyle's and Henry's laws explain why, as a diver descends while breathing compressed air: 1) inhaled PO2 and PN2 increase (Figure 1); and 2) the amount of nitrogen and oxygen entering the blood and tissues also increase.
Figure 1. Henry's and Dalton's laws predict that, with descent, inhaled PO2 and PN2 will increase and cause an increased amount of nitrogen and oxygen to enter the blood and tissues. The opposite occurs on ascent: inhaled PO2 and PN2 decrease, and allow the excess nitrogen and oxygen to leave the blood and tissues. IS THERE RISK OF OXYGEN TOXICITY IN DIVING? Since the pressure of all inhaled gases increases with increasing depth, there is risk of inhaling too much oxygen and developing oxygen toxicity. However, this is unlikely to occur in recreational diving because the depth limit (130 feet) effectively limits length of exposure to high oxygen concentrations. The recreational diver is exposed to high oxygen pressures, but not long enough to risk oxygen toxicity. The problem is a potential hazard at greater depths, or when breathing mixtures containing more than 21% oxygen (e.g., nitrox). Oxygen toxicity is discussed further in Section H. HOW DOES NITROGEN DIFFER FROM OXYGEN AND CO2? Unlike oxygen and carbon dioxide, nitrogen (N2) is inert; it is not metabolized by the body. At sea level the amount of N2 inhaled and exhaled is the same. This is not the case for O2 and CO2, which are not inert gases but instead participate in metabolism; as a result less O2 is exhaled than inhaled, and more CO2 is exhaled than inhaled. When breathing compressed air at depth, more gas molecules of air are inhaled because the air is at a higher pressure, and hence denser, than at sea level. Both the pressure and amount of inhaled nitrogen and oxygen are greater at depth than at sea level. Most of the extra oxygen is metabolized and doesn't pose any problem at recreational depths. But what about nitrogen, which is inert? The extra nitrogen that is inhaled has nowhere to go but into the blood and tissues, where it is stays in the gas phase ("dissolved") at the higher pressure, until the ambient pressure is reduced; then it starts to dissolve back out, and is excreted in the exhaled air. Two important problems relate to the increased quantity and pressure of nitrogen from inhaling compressed air: nitrogen narcosis and decompression sickness. Although both problems are related to too much nitrogen, they are distinct. Nitrogen narcosis is a function of the increased pressure of the gas and is only a problem as long as that pressure remains elevated. Thus nitrogen narcosis is solely a function of depth (see Chapter 9). For some divers narcosis begins to manifest in the 80-130 fsw range, for others much deeper. Because the problem is related only to depth, it can be cured by ascent. Although divers have succumbed from nitrogen narcosis, deaths appear to be due to drowning or failing to ascend (due to confusion), or to running out of air. Those who ascend escape the effects of nitrogen narcosis and recover completely. Decompression sickness (DCS) is not due to the pressure of nitrogen at depth per se, but instead to the formation of bubbles as dissolved nitrogen comes out of the tissues with ascent. Since bubble formation is in part related to the total amount of nitrogen in the tissues, the dive time is important (the longer the dive, the more nitrogen enters the tissues, up to a point). Thus DCS is a function of depth and duration of the dive and, at least among recreational divers, is a far more common problem than nitrogen narcosis. Unlike nitrogen narcosis, DCS can lead to permanent physical impairment. WHAT IS THE HISTORY OF DECOMPRESSION SICKNESS? Decompression sickness was appreciated as early as the mid-19th century, among bridge-building caisson workers. A caisson is a huge enclosed space sunk to the bottom of a lake or river, and pressurized with air to keep out water. Its use dates from the early 19th century. Men work inside a caisson while excavating for bridge foundations. By the mid-19th century it was observed that length of exposure to the increased air pressure in the caisson, and the worker's speed of ascent, correlated with development of joint pains after surfacing Ä then called "the bends." Afflicted men reminded some of the posture affected by fashionable women of the time, the 'Grecian Bend.' These women, with their bustles and full-length skirts, walked slightly stooped or 'bent over.' Of course the caisson workers were not just posturing; they were in real pain. The bends was a well recognized problem during construction of the Brooklyn Bridge in the 1870s. In 1878 Paul Bert, an eminent French physiologist, published his classic work Le Pression Barometrique, in which he recommended slow ascent in order to prevent the bends (Bert 1878). Over the next decade "Caisson Disease" became a recognized malady, and slow ascent from the caisson's high pressure was accepted as the method of prevention. In his 1892 edition of The Principles and Practice of Medicine, an important medical textbook of the era, Dr. William Osler devoted a full page to 'Caisson Disease'. Osler's clinical description cannot be improved upon today. He wrote: This remarkable affection, found in divers and in workers in caissons, is characterized by a paraplegia, more rarely a general palsy, which supervenes on returning from the compressed atmosphere to the surface... The symptoms are especially apt to come on if the change from the high to the ordinary atmospheric pressure is quickly made. They may supervene immediately on leaving the caisson, or they may be delayed for several hours. In the mildest form there are simply pains about the knees and in the legs, often of great severity, and occurring in paroxysms. Abdominal pain and vomiting are not uncommon. The legs may be tender to the touch, and the patient may walk with a stiff gait. Dizziness and headache may accompany these neuralgic symptoms, or may occur alone. More commonly in the severe form there is paralysis both of motion and sensation, usually a paraplegia but it may be general, involving the trunk and arms...In the most extreme instances the attacks resemble apoplexy, and the patient rapidly becomes comatose and death occurs in a few hours. In the cases of paraplegia [paralysis below the trunk] the outlook is usually good, and the paralysis may pass off in a day, or may continue for several weeks or even for months. Identical features are met with in the deep-sea divers. The explanation of this condition is by no means satisfactory... It has been suggested that the symptoms are due to the liberation in the spinal cord of bubbles of nitrogen which have been absorbed by the blood under the high pressure... A large majority of the cases recover. The severe neuralgic pains often require morphia [morphine]. Inhalations of oxygen and the use of compressed air have been advised. When paraplegia develops the treatment is similar to that of other forms. In all caisson work care should be exercised that the time in passing through the lock from the high to the ordinary pressure be sufficiently prolonged. Early in this century the Scottish physiologist John Scott Haldane and colleagues, in experiments with goats and humans, worked out safe decompression tables for divers (Boycott 1908; see Sections A and J). Their calculations, based on half times for nitrogen in various parts of the body, formed the basis for navy dive tables still in use today. WHAT CAUSES DECOMPRESSION SICKNESS? Henry's and Dalton's Laws predict that, as the diver descends, excess nitrogen will enter the blood and all body tissues. These laws also predict that, on ascent (as ambient pressure decreases) the extra nitrogen that accumulated will diffuse out of the tissues and into the circulation. Decompression sickness (DCS) arises when excess nitrogen leaving tissue forms bubbles large enough to cause symptoms. Size of bubbles is important, since small bubbles can often be found in divers with no symptoms (detection of bubbles is with Doppler ultrasound). DCS arises when the pressure gradient for nitrogen leaving the tissues is so great that large bubbles form, probably by coalescence of many smaller bubbles. Large bubbles within tissues and the circulation cause the symptoms and signs of decompression sickness. When nitrogen bubbles leave the tissues they first enter capillaries and then the veins. Veins (venous circulation) leave the tissues and return to the heart and lungs in order to pick up fresh oxygen. After going through the lungs, the blood enters the arterial circulation (see Section C). Nitrogen bubbles travel in the venous circulation to the lungs, but then (in people with normal anatomy) are trapped in the lung capillaries because the bubbles are larger than the tiny diameter of the capillaries. Once trapped, the bubbles break up and the nitrogen gas is exhaled. As a result of being trapped and exhaled, the bubbles do not enter the arterial circulation. However, DCS bubbles can bypass the lungs through an abnormal opening in the heart, called a patent foramen ovale (PFO; discussed later in this chapter). PFO, a relatively new area of investigation, may explain why some people with DCS apparently have nitrogen bubbles in the arterial circulation. Because any of the body's tissues can be affected by nitrogen bubbles, DCS symptoms are wide-ranging: from skin mottling to mild tingling in the hands or feet to shock and death. Blockage of blood flow to joints by the bubbles causes pain, which is "the bends." Blockage of blood flow to nervous tissue can cause paralysis or stroke. There are several theories as to why bubbles form in the first place. In a pure, static fluid such as blood in a beaker that undergoes sudden decompression, bubbles don't form. Why they form in people (and animals) may have something to do with excess nitrogen entering "gas nuclei," sub-microscopic pockets of gas that are said to exist naturally. There are other theories, all too complex to bother with here. Whatever the exact mechanism of bubble formation, the following statements reflect current understanding of decompression sickness. Decompression causes excess nitrogen to leave tissues and enter the blood stream, from where it travels to the lungs and is exhaled. Nitrogen leaves the tissues either dissolved in the blood or in bubbles. The dissolved state is harmless whereas nitrogen bubbles, because they are space-occupying, can compress nerves and/or block the circulation of blood. Bubbles can also cause some chemical reactions in the blood which are harmful to the body. The larger and more numerous the nitrogen bubbles, the more likely they will cause symptoms of DCS. For a given individual, DCS is unpredictable. Its occurrence depends in large part on the recent diving history (i.e., profiles of the preceding dive(s), including rate(s) of ascent), and also on individual host factors, including age, amount of body fat, state of hydration, and individual susceptibility in ways that cannot be quantified. DCS is sometimes divided into Type I, which encompasses the bends and skin manifestations, and Type II, which includes pulmonary, central nervous system and cardiovascular problems, i.e., the more serious manifestations of DCS (see Section G, Table 2). This division is more useful for retrospective analysis and is less helpful for determining outcome, since many patients with DCS present with a range of symptoms (Green 1987). Also, in some patients Type I symptoms may later progress to Type II symptoms. Neurologic symptoms are particularly common because nitrogen is highly soluble in fat (five times more than in blood), and dissolves readily in the fatty myelin sheaths that surround nerves. As the diver ascends nitrogen comes out of these nerve sheaths; if too much nitrogen is in these nerve sheaths at the beginning of ascent, bubbles may form and compress nerves even before they enter the venous circulation. Apart from compressing nerves and blocking circulation, bubbles can also set off certain chemical reactions, collectively called an "inflammatory response." An inflammatory response is marked by release of certain protein compounds that can damage the blood vessels and affect blood clotting (Green 1987). Although this process is poorly understood in decompression sickness, it is important to note that chemical changes do occur in the blood of DCS patients, and that some symptoms are not simply the result of nerve compression or circulation blockage (Bove 1982, Catron 1982, Smith 1994). IS "THE BENDS" THE SAME THING AS DCS? No. Strictly speaking, "the bends" is a slang or popular term for the "pain only" manifestations of DCS, a Type I DCS. The pain is usually in the joints, typically in the shoulders or elbows first, but can occur virtually anywhere in the body. Other manifestations of DCS, including skin mottling and itching (also Type I), and all Type II problems (paralysis, lung edema, shock, etc.) are not "the bends." Less strictly speaking, the term "bends" is sometimes used as a synonym for any manifestation of DCS, or for all DCS-related problems. Thus, the phrase "He got bent" may refer to a diver who developed pain only, who developed pain followed by paralysis, or who died. This is not medical terminology, and physicians reporting on the patient would use more precise language. However, in everyday conversation "bends" and "bent" usually serve to indicate some problem arising from DCS. WHAT ARE THE DIFFERENCES BETWEEN DCS AND AGE? In contrast to air embolism, DCS commonly presents gradually, starting as tingling and numbness (Table 1). The median time of onset for the first symptoms of DCS is about 20 minutes after surfacing; for the next symptom, 2 hours. By contrast, AGE almost always presents within the first 10 minutes after surfacing from a dive (DAN 1988). Potential neurologic complications of DCS include stroke and spinal cord paralysis, the latter most likely from occlusion of the blood vessels surrounding the spinal cord. Of 117 symptomatic divers in one study, 70 were judged to have neurologic decompression sickness and 39 air embolism (Dick 1985). After paresthesia (skin tingling), the most common neurologic decompression symptoms were limb paralysis, vertigo, unsteady gait, mild headache, and blurred vision. Of 133 patients treated for DCS in Englandover a 20 year period, 81% presented with spinal cord injury, 27% with cerebral injury, and 10.5% with inner ear symptoms (Green 1987). Shortness of breath from DCS, usually referred to as the "chokes," is much less common than musculoskeletal and neurologic complaints (Dewey 1962; Green 1987). Symptoms of chokes include pain under the breast bone, cough and shortness of breath. The pain is often intensified with a deep breath, and may seem worse when taking in a breath. The cough is generally non-productive but on occasion the patient may cough up some blood. Chokes is due to movement of many nitrogen bubbles from the venous system to the lungs. Although a small number of bubbles will be trapped and exhaled, a large number can overwhelm the lung circulation, and lead to respiratory distress. Symptoms can occur up to 12 hours after the dive and may persist for 12-48 hours. In severe cases pulmonary edema (fluid in the alveolar spaces) can result from the chokes (Strauss 1979).
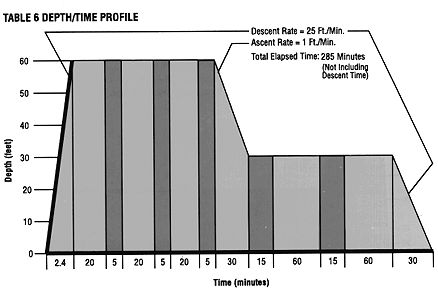
The only effective treatment for DCS (and AGE) is recompression in a hyperbaric chamber, the sooner the better. All manifestations of DCS are potentially reversible if the victim can be quickly recompressed in a chamber. Recompression squeezes the nitrogen bubbles to a smaller size and allows a slower and safer egress of nitrogen from the tissues. Delay in hyperbaric therapy may result in permanent paralysis. Treatment is recommended even if symptoms abate or clear before the patient reaches the chamber. This is because bubbles may still be present in the circulation, and could lead to a more devastating problem later on. Hyperbaric chambers can be found in or near most large U.S. cities (see Appendix A). Although altitude can worsen decompression sickness by lowering ambient pressure and increasing nitrogen egress from the tissues, time saved by flying to a chamber generally outweighs the risk. Ideally the transport aircraft should be pressurized to sea level, or else fly as close to the surface as feasible (less than 1000 feet altitude if possible). In many cases the most costly aspect of treating decompression sickness is transporting the victim to a hyperbaric chamber. A hyperbaric treatment table for DCS is shown in Figure 2. Whether or not to use this particular table, and how often, is always up to the physician evaluating the stricken diver. Also, any complications during treatment (such as oxygen toxicity) could lead to modifications of the schedule. Some physicians also recommend corticosteroids in DCS to decrease inflammation, but their use is largely anecdotal and efficacy is unproven (Catron 1982). Various other drugs, such as heparin, diazepam, aspirin, and vasodilators, have been studied or used in this condition (Catron 1982); none can be routinely recommended as effective. Except for oxygen, which can be considered a drug, no particular drug is helpful for DCS.
Figure 2. U.S. Navy Table 6 for Treatment of DCS. Light-shaded areas represent periods of 100% O2, dark-shaded areas periods of air breathing. Recompression treatment is usually effective if begun early but the major emphasis, of course, should be on prevention: using common sense and staying well within established limits for each dive. WHAT IS DECOMPRESSION ILLNESS? Admittedly, doctors have a way of confusing things at times. Because DCS and AGE are treated basically the same way (by recompression in a hyperbaric chamber), and their root cause is the same (gas bubbles in some part of the body), many hyperbaric physicians feel there is little point in trying to distinguish between the two conditions; they prefer to call all bubble disease in divers by one name. I agree with this preference. Unfortunately, the name chosen to cover both forms of bubble disease is "decompression illness." I say unfortunate because the term is too close to "decompression sickness," which specifically means nitrogen bubble disease from too rapid decompression and not AGE, which is from barotrauma. Why choose a name almost identical to one type of bubble disease (DCS), to refer to all bubble disease (DCS + AGE)? Well, I didn't choose the name, and I hope someone or some organization changes it. A better name would be diver's bubble disease (DBD), or some other general term to encompass both conditions. I mention all this because some authors now use the term DCI without clearly explaining that they mean both DCS and AGE. One textbook, otherwise excellent in its explanations, mistakenly calls DCI a "unique condition" characterized by "AGE followed by DCS." It is even conceivable that someone will use the term DCI to mean only DCS (after all, 'illness' and 'sickness' are synonyms). Well, you get the (confusing) picture. After decades of using 'decompression sickness' to denote nitrogen bubble disease only, the term 'decompression illness' for the entire gamut of bubble problems is bound to be confusing. Keep in mind that although the clinical manifestations and treatment of DCS and AGE are similar, the underlying mechanisms are different. At the very least, all authors should clearly define whatever term they do use. WHAT IS A PATENT FORAMEN OVALE AND WHAT IS ITS SIGNIFICANCE IN SCUBA DIVING? The foramen ovale is an opening between the right and left atria of the heart that normally closes shortly after birth. Although the right and left atria share a common wall, after birth there is no direct communication between them. Oxygen-depleted (venous) blood from the entire body enters the right atrium and then goes to the adjacent right ventricle (see Section C). From the right ventricle blood goes to the lungs, where O2 is taken up and CO2 given off. The oxygen-rich blood then travels to the left atrium and left ventricle, from where it is pumped out to the arterial circulation and all the body's organs. In a certain percentage of people the foramen ovale does not close completely at birth, but stays open. Using a variety of methods, some opening can be demonstrated in 20-30% of adults; this situation is called a patent foramen ovale or PFO. A PFO generally causes no problems, as it is small and little or no blood actually passes through the opening. Sometimes an opening is only demonstrated with a transient increase in right atrial pressure, such as during a Valsalva maneuver (or when a diver clears her ears). Thus, in most people with a PFO, the normal flow of blood is still maintained. In a small percentage of people with a PFO, however, the opening is large and a significant amount of blood is "shunted" from the right to the left atrium, thus bypassing the lungs. Any opening through the heart that lets venous blood bypass the lungs could cause problems. Since Doppler studies have demonstrated venous gas bubbles on ascent from most dives, we infer that the lungs are vital to filtering out these "silent bubbles"; the bubbles break up in the pulmonary capillaries so the nitrogen dissolves harmlessly into the blood. Thus the lungs help prevent small venous bubbles incurred in diving from entering the arterial circulation. A large patent foramen ovale allows nitrogen bubbles to pass from the right to the left atrium, thus bypassing the lungs and entering the arterial circulation (Figure 3). If these shunted bubbles are in sufficient size and/or number, they can cause organ damage. This is the theory, and only in recent years has evidence accumulated about the risks from a PFO when scuba diving. Like most situations in diving, however, the risk is a matter of degree; the larger the PFO, and the more bubbles that exist in the venous circulation, the more likely will bubbles bypass the lungs and enter the arterial circulation.
Figure 3: Foramen Ovale. When closed (normal), venous bubbles go from right atrium to right ventricle of the heart and then into lungs where they are trapped by the lung capillaries and do not enter the arterial circulation. When open (patent), venous bubbles go from right atrium through the patent foramen directly into the left atrium; from there, they enter the left ventricle and the arterial circulation, where they may cause symptoms similar to AGE. One study, using bubble contrast echocardiography, demonstrated shunting through a PFO in 11 of 30 divers (26 recreational, 4 professional) treated for DCS (Moon 1989); this percentage was statistically higher than the prevalence of PFO in a control group, suggesting PFO can be a risk factor for symptomatic DCS. Eighteen of the patients had serious signs and symptoms, defined as motor weakness, dizziness, or cognitive impairment (confusion, impairment of consciousness); presumably these symptoms arose from venous bubbles traveling to the brain instead of being trapped by the lungs. Another nine patients had only sensory abnormalities, with or without musculoskeletal pain, and 3 had only pain. Another study, in the British Medical Journal, reported MRI brain scanning in 87 volunteer recreational divers (minimum 160 dives each) and without symptoms. The study showed multiple brain "lesions" when there was a large PFO (Knauth 1997). The size of the PFO was defined by the degree of blood shunting through the atrial opening. A brain lesion was defined as a hyper-intense "bright" spot seen on the MRI scan. Several sport divers with a large shunt through their PFO had many more MRI bright spots than did divers without a shunt. Unlike the divers in the paper by Moon, et.al., however, this group had no history of DCS and no symptoms, so the clinical significance of the bright spots is unknown. However, the implication of the MRI findings is clear: venous bubbles at some point did pass through the PFO shunt to directly enter the arterial circulation. From this and other reports (Wilmshurst 1989; Cross 1989; Wilmshurst 1992; Johnston 1996; Wilmshurst 1996; Wilmshurst 1997) it is now apparent that:
The potential for damage from venous bubbles in the arterial circulation is the same as from air bubbles of arterial gas embolism. Thus the two types of bubbles have different origins (nitrogen egress for DCS, barotrauma for AGE), but the appearance of the patient can be quite similar. This fact highlights the importance of a unified approach to any bubble problem. While the information outlined in Table 1 is correct in principle, many diving accidents caused by bubbles cannot be diagnosed as definitively DCS or AGE.
SHOULD DIVERS BE CHECKED FOR A PATENT FORAMEN OVALE? SHOULD DIVERS WITH A PATENT FORAMEN OVALE NOT SCUBA DIVE? Patent foramen ovale is something that might be looked for after a diving accident when there are neurologic symptoms (e.g., Type II DCS), to explain the possible mechanism; this is particularly important if the injured diver stayed within acceptable depth and time limits. However, at present there is no general recommendation that all sport divers have a test for patent foramen ovale. The technique to demonstrate it is costly and, more importantly, finding a small PFO does not prove the diver is at any increased risk for a decompression problem. There are millions of recreational scuba divers. If a PFO can be found in 25% of scuba divers when carefully looked for, and there are only a few hundred cases of DCS reported yearly, it seems highly unlikely that demonstration of PFO would indicate any special risk for DCS. Furthermore, in the study by Knauth, et. al., the significant brain lesions occurred only in some of the divers with PFO and a large shunt. Finally, none of the divers with brain lesions had a history of DCS. Like many other issues in diving, this one is unsettled and somewhat controversial (Wilmshurst 1992; Cross 1992; Wilmshurst 1997). As Wilmshurst pointed out in an editorial accompanying the MRI article:
If a diver is known to have a PFO with a large right to left shunt, current knowledge suggests it would probably be prudent to refrain from diving until the opening is repaired (Wilmshurst 1996). At the least, the diver should consult a cardiologist knowledgeable about scuba diving. PFO and diving is an evolving area of research, and anyone interested in the subject should stay tuned for new studies that will surely be done on this important subject.
WHY IS IT UNSAFE TO FLY SOON AFTER DIVING? It is 1 p.m. and Anne has just completed her sixth Caribbean dive over a 3-day period. She boards her plane home at 7 p.m. the same day. Twenty minutes into the flight Anne begins to have severe pain in both elbows and feels achy all over. She thinks she's coming down with the flu, and is thankful it didn't happen while she was diving. She takes two aspirin but the elbow pain persists. Two hours later, on landing, she feels much better, and attributes improvement to the aspirin. Anne actually suffered a classic episode of type I DCS ("bends") while flying. Had she not boarded the plane so soon after diving she would have developed no elbow pain or flu-like ache. Dive tables are designed to help prevent episodes of DCS. Unless specifically designed for altitude diving, the tables assume the diver will surface at or close to sea level. The tables assume the diver will experience a controlled decompression from some water depth to sea level, so the excess nitrogen can be safely unloaded. If the diver instead goes to an ambient pressure significantly less than sea level, the tables are no longer valid. Flying always presents an ambient pressure less than sea level. Flying in effect represents a second decompression, this time between sea level and the cabin altitude. The airplane cabin, although pressurized, is still at a much lower pressure than sea level. At 30,000 feet altitude, the airplane cabin on commercial airliners is usually pressurized to between 7000 and 8000 feet. In fact everyone in the plane experiences some decompression, but the amount of nitrogen lost by people who haven't been diving is trivial and causes no symptoms. (Jet fighter pilots who very rapidly ascend from sea level to high altitude can get the bends for the same reason as divers: too rapid ascent causing nitrogen bubbles to form in the tissues and blood.) Fly too soon after surfacing from a dive and the further reduction in ambient pressure can result in excess nitrogen forming bubbles large or plentiful enough to cause DCS. It is universally recommended that you wait some time after diving before flying. HOW LONG SHOULD A DIVER WAIT BEFORE FLYING? The answer to this question is both simple and complex. The simple answer is to wait until the excess nitrogen has off-loaded enough so that you won't develop the bends at altitude. That length of time will depend on how many dives have been made over what period of time, the dive profiles, and how many hours have elapsed since the last dive; all this information makes the answer complex, and, ultimately, unique to the individual diver. DAN and other organizations have, over the years, made general recommendations applicable to various diving activities, e.g., repetitive recreational dives, single recreational dives, decompression dives, etc. DAN's recommendations for recreational divers include the following (as printed in Alert Diver, May/June 1994): 1. Divers making single dives per diving day, should have a minimum surface interval of 12 hours before ascending to altitude. This includes going to altitude by aircraft, automobile or any other means. 2. Divers who make multiple dives per day or over many days, or divers that require obligated decompression stops should take special precautions and wait for an extended surface interval beyond 12 hours before ascending to altitude. Extended surface intervals allow for additional denitrogenation and may reduce the likelihood of developing symptoms. For those diving heavily during an extended vacation, it may not be a bad idea to take a day off at midweek, or save the last day to buy those last-minute souvenirs. As a general rule, 24 hours after multiple dives is a safe waiting period. A dive computer will also display a time considered safe to fly after repetitive diving, and this may be less than 24 hours. However, in the event of computer failure at any point, always revert to the DAN guidelines. When in doubt, it seems prudent to wait a full 24 hours before flying.
Answers to TEST YOUR UNDERSTANDING1. When air is compressed the percentage of each individual gas remains the same; hence the percentage of oxygen in compressed air is always .21 (21%) regardless of depth. The partial pressure of inhaled oxygen (PO2) does increase with an increase in ambient pressure; at 33 fsw PO2 will be .42 atm. O2; at 66 fsw PO2 will be .63 atm. O2, etc. Because the gas is compressed the number of gas molecules inhaled will increase as well. 2. At sea level the partial pressure of oxygen is .21 x 760 mm Hg = 159.6 mm Hg. Since 33 fsw = 2 atm. total pressure, the PO2 of inspired air at this depth is 2 x 159.6, or 319.2 mm Hg. At 66 fsw the PO2 of inspired air is 3 x 159.6, or 478.8 mm Hg. 3. At sea level the partial pressure of nitrogen is .78 x 760 mm Hg = 592.8 mm Hg; this is also .78 atm. N2. At 33 fsw the PN2 is 2 x 592.8 = 1185.6 mm Hg, or 1.56 atm. N2. At 66 fsw the PN2 is 3 x 592.8 =1778.4 mm Hg, or 2.34 atm. N2. 4. If a certain percentage of tank air is a gas contaminant (e.g., 1%), that percentage will be unchanged at whatever depth the air is inhaled. However the pressure of the contaminant, just like the component gases of air, will equal the surrounding or ambient pressure, which at 66 fsw is 3x atmospheric. Since 1% of sea level air pressure is 7.6 mm Hg, the contaminant will have a pressure of 3 x 7.6 mm Hg, or 23.8 mm Hg. 5. a. AGE b. DCS c. Most likely DCS 6. a. T ---> V ---> R ---> L ---> A 7. a. True b. False c. False d. False 8. Many times diagnosis will depend on the details of the dive profile and post-dive history. Two aspects of this real case history argue against DCS as the diagnosis. First, there were no symptoms on the plane. Since high altitude expands any bubbles in the body, the lack of symptoms during flight suggests no significant amount of bubbles were remaining at the time (i.e., that she adequately decompressed by waiting 24 hours). Second, five days after the last dive is well outside the time for presentation of DCS. The same symptom appearing hours after her last dive would have been highly suggestive of DCS, and may have lead to chamber re-compression. Presenting as it did, however, the chance of DCS being the cause was very remote. She was referred to her family physician and the problem resolved spontaneously. REFERENCES AND BIBLIOGRAPHY SECTION G.Effects of Increased Dissolved Nitrogen from Scuba Diving: Decompression SicknessArthur DC, Margulies RA. A short course in diving medicine. Annals Emerg Med 1987; 16:689-701. Boettger ML. Scuba diving emergencies: pulmonary overpressure accidents and decompression sickness. Annals Emerg Med 1983;12:563-567. Boycott AE, Damant GCC, Haldane JS. The prevention of compressed-air illness. J Hyg Camb 1908;8:342-443. Bove AA. The basis for drug therapy in decompression sickness. Undersea Biomed Res 1982;9:91-111. Butler BD, Laine GA, Leiman BC, et al. Effect of the Trendelenburg position on the distribution of arterial air emboli in dogs. Annals Thor Surg 1988;45:198-202. Catron PW, Flynn Et, Jr. Adjuvant drug therapy for decompression sickness: a review. Undersea Biomed Res 1982;9:161-74. Cross SJ, Thomson LF, Jennings KP, Shields TG. Right-to-left shunt and neurological decompression sickness in divers. (Letter). Lancet 1989;ii;568. Cross SJ, Lee HS, Thomson LF, Jennings K. Patent foramen ovale and subaqua diving (letter). BMJ 1992;304:1312. Colebatch HJH, Smith MM, Ng CKY. Increased elastic recoil as a determinant of pulmonary barotrauma in divers. Resp Physiol 1976;26:55-64. Davis JC, Kizer KW. Diving Medicine. In: Auerbach PS, Geehr EC, editors. Management of Wilderness and Environmental Emergencies, 2nd edition. The C.V. Mosby Co., St. Louis, 1989. Dewey AW, Jr. Decompression sickness, an emerging recreational hazard. N Engl J Med 1962: 267:759-65; 812-20. Dick APK, Massey EW. Neurologic presentation of decompression sickness and air embolism in sport divers. Neurology 1985; 35:667-671. Edmonds C. Barotrauma. In Strauss R., editor. Diving Medicine. New York, Grune & Stratton, 1976. Green RD, Leitch DR. Twenty years of treating decompression sickness. Aviat Space Environ Med 1987;58:362-6. Gorman DF. Decompression sickness and arterial gas embolism in sports scuba divers. Sports Medicine 1989;8:32-42. Johnston RP, Broome JR, Hunt PD, et. al. Patent foramen ovale and decompression illness in divers (letter). The Lancet 1996; 348: 1515. Kindwall EP. Diving emergencies. In: Kravis TC, editor. Emergency Medicine; Aspen Systems Corporation, Rockville, Maryland, 1983. Kizer KW. Dysbaric cerebral air embolism in Hawaii. Ann Emerg Med 1987;16:535-41. Knauth M, Ries S, Pohimann S, et. al. Cohort study of multiple brain lesions in sport divers: role of a patent foramen ovale. BMJ 1997; 314:701-703. Krzyzak J. A case of delayed-onset pulmonary barotrauma in a scuba diver. Undersea Biomed Res 1987;14:553-61. Macklin MT, Macklin CC. Malignant interstitial emphysema of the lungs and mediastinum as an imporant occult complication in many respiratory diseases and other conditions: An interpretation of the clinical literature in the light of laboratory experiment. Medicine 1944;23:281-358. Mebane GY, Dick AP. DAN Underwater Diving Accident Manual. Divers Alert Network, Duke Univesity, 1985. Moon RE, Camporesi EM, Kisslo JA. Patent foramen ovale and decompression sickness in divers. Lancet 1989;1:513-514. Neblett LM. Otolaryngology and sport scuba diving. Update and guidelines. Annals Otology, Rhin and Laryng. Supplement 1985; 115:1-12. Orr D. Know When to Say When. Judging your risks before flying after diving is your own informed decision. Alert Diver, May/ June, 1994; p. 13. Osler W. The Principles and Practice of Medicine, D. Appleton and Co., New York; 1892. Page 827. Roydhouse N. 1001 disorders of the ear, nose and sinuses in scuba divers. Can J Appl Spt Sci 1985;10:99-103. Schaefer KE, McNulty WP Jr., Carey C, Liebow AA. Mechanisms in development of interstitial emphysema and air embolism on decompression from depth. J Appl Physiol 1958;13:15-29. Strauss RH. Diving Medicine: State of the Art. Amer Rev Resp Dis 1979;119:1001-1023. Weeth JB. Management of underwater accidents. JAMA 1965; 192: 215-219. Wilmshurst P, Byrne JC, Webb-Peploe MM. Relation between interatrial shunts and decompression sickness in divers. Lancet 1989;II;1302-1306. Wilmshurst. Patent formen ovale and subaqua diving (letter). BMJ 1992;1312. Wilmshurst P. Transcatheter occlusion of foramen ovale with a button device after neurological decompression illness in professional divers. The Lancet 1996;348:752-753. Wilmshurst P. Brain damage in divers (editorial). BMJ 1997; 314: 689-690. |
|||||||||||||||||||||||||||||||||||